This is a follow-up of Experiment: Can an externally heated object actually heat its heat source?
Which stemmed from: Global warming/climate change .
Intro
To recap the question I wanted to see for myself is, is it indeed impossible to heat a heat source by reflecting its own heat back at it, or having it heat another object that then heats it in turn?
Although it’s obviously the case from lived experience – just picture standing in an aluminum-lined closet, you don’t fear you will burst into flame, will you? – I wanted to mess around with it myself and see how these things work practically.
So I messed around a lot, got an adjustable-output DC power supply (anywhere from 0.001V to 31.00V with 0.0001A to 5.200A), some nichrome wire (resistive heating wire), some thermocouples, and a vacuum chamber, and…
I never was able to even get close to the theoretical blackbody maximum for what the wire should be, let alone higher than that.
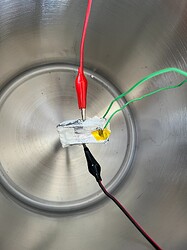
The Setup
The case study is: a 12.3cm length of nichrome wire with diameter 0.5mm.
This has surface area pi * 0.5mm * 123mm + pi*(0.25mm)^2*2 = 193.6 mm^2 .
Plugging it into the circuit, at 1.00V input it runs 1.364A through the wire, which equals a total input of 1.364 W.
The nichrome wire’s emissivity is 0.95 or so (it’s oxidized), so the blackbody theoretical maximum is 328.2°C.
Bare Wire
The wire looked like this:
If I touch a thermocouple to the wire itself it gets to… 120°C.
Sandwiched Wire
Ok perhaps the thermocouple isn’t best attached that way, so I sandwich it between two thin glass plates (20mm x 20mm x 0.15mm) and tape the sides, stick the thermocouple in between the wire and… 121.1°C.
Tape it Up
Blowing on it cools it so there’s convection loss at play. If I wrap the thing in a ton more thermal tape… 131.4°C. If I touch a 2nd thermocouple to the outside of the glass it’s ~72°C.
Foil it Up
If I now wrap this in aluminum foil as well… aluminum foil reflects 97% of the infrared light coming to it. At 131.4°C it would be emitting 0.28 W. By the global warming logic 97% of this will be reflected , so we would add 0.27W to the input and now it will be emitting 0.28+0.27=0.55W or get to 206°C. Now this 0.55W also would be reflected 97% adding another 0.53W, so then it emits 0.28+0.53=0.81W or get to 254°C, etc etc until it finally reaches a peak at 9.3W (9.3W * 0.03 that the aluminum lets through = the 0.28W of initial input).
So by the exact same logic as the greenhouse effect, the inside should be 9.3W while the outside continues being 0.28W, all with a total 1.3W input – and somehow this doesn’t violate any thermodynamic laws. In any case as I’m measuring the inside, at 9.3W the temp should now be 698.6°C.
The result looked like this:
And what did I get? … … … 146.1°C, with a thermocouple attached to the outside wrapped in foil getting to 93.8°C . This 97% reflection barely bumped it up to a blackbody equivalent of 0.32W emitting , or +0.04W.
GHE Proven??
“Ahhh!” One might say. “But the principle is proven, the wire’s own heat was reflected back, heating it further, if only by a little bit!”
Yet this doesn’t show that. Why? Because in the real world you have to measure things, and there are a lot of variables at play. The thermocouple doesn’t magically heat up – it has to heat up by getting heat from the heat source. For example if I run enough current to cause the wire to visibly glow, albeit just a bit, and touch the thermocouple to it – the part that it’s touching gets to a visibly cooler temperature. The heat conducts from the wire to the thermocouple.
So what does it mean the thermocouple got hotter wrapped with thermal tape and foil? It can fully be explained by the initial heat from the heat source (which can get up to 328°C given the power input) that the thermocouple didn’t initially absorb, being reflected back so the thermocouple does absorb it. Basically it increases the consistency or efficiency of the heat distribution of the initial heat. The initial heat is distributed better — but it isn’t heating the heat source up.
For a similar example see: Slaying Watts with Watts | Climate of Sophistry .
BTW the thermocouple itself is a sphere ~1mm in diameter:
This has 6.29mm^2 surface area, so, it only negligibly affects the blackbody calculation. (328.2°C → 323.4°C).
Vacuum it Up
“Ok”, one might say, “but this is open air, not a vacuum. Convection is messing with it!” Ok, so I got a vacuum chamber, stuck it all in the vacuum, used playdoh to be able to seal the chamber with wires going down it, and the new temperature is… drumroll… 180.8°C!
Not even close to +320°C, let alone higher.
By comparison, without the foil, but in the vacuum chamber, it gets to 168.6°C.
Magic Foil Sphere
Ok, but the foil was directly wrapped around the taped glass. The ‘magic’ formula is supposed to be only with radiative heat loss, no conduction or convection. So, I took off the foil, then I created a foil sphere that wouldn’t touch the wire or taped inside anyway whatsoever. It was really more a blob than a sphere ![]()
With no vacuum it got to 155.5°C — more than the foil wrap. This would be due to prevention of convective heat loss.
In the vacuum and … … 178.1°C. Hmm less than the foil wrap?
Well there were many holes and it wasn’t well-sealed, so I taped over the holes, sealed it better, re-run it again through the vacuum and … … … 190.5°C!
More than before but… still nowhere close to even the basic blackbody peak. And the full blackbody prediction with the foil should be a 1.364W source / 0.03 = 45.47W or 1,178°C emitted to have “power out” equal “power in”!
Magic Foil Box
Ok maybe the foil sphere was too loosey-goosey? And maybe the glass absorbed too much heat? And the wire was all on a horizontal plane, what if I curl it around itself more?
So I did all this, took the glass and tape off, redid the wire to be more coiled, made a small cardboard box with each inside face being wrapped in foil, made sure nothing touching the wire, and stuck the the thermocouple snugly inside the middle of it with some small amount of thermal tape to keep it inside.
Result:
Ran it without a vacuum and it got to … … 186.2°C! More than the foil-wrapped one from within vacuum before!
It was still going up a bit but had mostly stabilized, so I turned on the vacuum and … 200.6°C!
A new peak, yet… still not even close to the 328.2°C. Remember to double the temperature (in Kelvin) you need to multiply the power by 16x. So it takes more and more energy as you go up in levels. To get from 473 K (200°C) to 601 K ( 32°C) you need to 2.6x the power.
Cardboard Box Control Run
Afterwards I unwrapped the foil of the box (took away the foil on the inside, added a new top) to do a test run:
With the GHE logic, this should lead to a doubling of power. The wire should initially be emitting 1.364W, which will then heat up the cardboard box. The cardboard box then emits this amount in two directions, 0.682W inside and 0.682W outside. This 0.682W is added to the 1.364W to get 2.047W, etc., which continues until the equilibrum is reached of the wire emitting 1.364/0.50 = 2.728W , which is 441°C.
The box got… 151.8°C in regular air. Ok ok again it’s the conduction/convection demons, let’s run it in the vacuum and… we get …179.9°C.
Interestingly the peak temperature in the vacuum chamber actually went up at a certain point as the air pressure increased – so in this configuration some amount of air present is beneficial, probably because it retains and diffuses the heat better within the box.
Summary of Results
So we have:
| open air | enclosed air | vacuum chamber | quantumville | |
|---|---|---|---|---|
| bare wire | ~120°C | N/A [1] | ||
| encased wire | 121.1 °C | N/A [1:1] | ||
| v1[2] (taped glass) | 131.4°C | 147.1°C | 168.6°C | 328.2°C [3] |
| v1 foil wrap | 146.1°C | 180.8°C | 328.2°C - 1,178°C [4] | |
| v1 foil sphere 1 | 155.5°C | 156.1°C | 178.1°C | 328.2°C - 1,178°C [4:1] |
| v1 foil sphere 2 | 190.5°C | 1,178°C [5] | ||
| v2[6] cardboard box | ~151.8°C+ | 179.9°C | 441°C [7] | |
| v2 foil box | ~186.2°C+ | 200.6°C | 1,178°C [5:1] |
Conclusion
In reality, the vacuum chamber reduces the convective heat loss (although doesn’t eliminate it), while the boxing and foiling more efficiently distributes the source heat.
We don’t ever get more energy out than energy in, either in net, or even redistributed temporarily “within”.
You Can’t Ignore Reality
Even within this tiny experiment there were so many different variables that actually affected things. For example if I added more tape then the peak temperature would decrease, because… there’s more material to be heated up? Higher surface area? Different emissivity of the tape? Different heat conductance properties? There’s any number of factors.
I can easily just draw a diagram with a wire, with 7,044 W/m^2 drawn upwards and downwards (equivalent of 328.2°C), then draw some more arrows that 97% of it is reflected back, do some math and come up with that I should get 234,800 W/m^2 upwards instead (1,178°C equivalent).
But it goes to show that, you can’t ignore conduction, convection, actual materials, real processes, to get an accurate prediction… not to mention the basic laws of physics
This is in a tiny vacuum chamber in one room with minimal amounts of material and power input. Now consider that an equivalently-detailed diagram is used to explain the basic GHE for AN ENTIRE PLANET’s worth of reality and… it might give a better sense of the scope of just how baseless such a starting point is!
GHE Disproved?
Does this experiment disprove the logic of the GHE – going from a -18°C maximum possible power input bringing itself up by self-heating to +15°C? I would say yes, with qualifiers.
Yes in that if it were remotely possible then we should see far higher temperatures than we do in the experiment – especially with the aluminum foil which reflects 97% of the IR light!
The qualifier is that there are various confounding factors I couldn’t fully eliminate:
- Circuit Loss: Some amount of power went into the rest of the circuit, and not the wire. However I figured this was around ~0.1W of the ~1.3W going into it – re-doing the Quantumville numbers with this figure instead, doesn’t change the conclusions. It also goes to show you can’t just take a number and apply it willy-nilly, reality is always more complex than that.
- Thermocouple Heating: As the thermocouple needs to be heated, then the wire itself might not be able to reach its max temp, due to needing to heat the thermocouple… but the surface-area of the heatable part of the thermocouple is just ~6mm^2 so it doesn’t affect the blackbody calculation much.
- Imperfect Vacuum: My vacuum chamber would get to ~0.03atm , or ~1/30th of the normal atmospheric pressure. This is a lot less than normal and certainly reduces convection a lot – but apparently you need to get down to ~0.001 atm to mark it fully negligible.
However in the cardboard box experiment at some point the steady-state temperature actually was higher with a bit more air in it. So, it’s not that simple. Further… the Earth itself actually has an atmosphere between the ground and the supposed magic CO2-bouncing-back-magnifying-effect layers. So it’s even besides the point to claim this magic doubling effect of radiative heat transfer only applies in a vacuum – the Earth’s surface-atmosphere system is most obviously not a vacuum.
To the extent the effect depends on only losing heat radiatively from the top, a more perfect vacuum would help… however that only seems relevant regarding how much the exterior heats up. The aluminum foil should still provide plenty of back radiation for the effect to work. - Conduction Along the Alligator Clips: The wire was clipped to the power source with alligator clips. So, some heat seeps out from the wire to the clips via conduction. That being said the wire has no issue maintaining a red-hot glow at higher wattages, and even so if I immediately touch the clip as soon as I turn the power off, even while the middle is red-hot I can still touch the clip and not get hurt.
So it does exist but it’s not necessarily overpowering, and further even so the energy that was still in the wire should still have had the multiplying effect it’s said to have with the GHE logic.
There may, of course, be other factors I didn’t consider. So someone can always say that I failed to account for x, y, or Z, that it doesn’t match up because of A or B reasons.
Burden of Proof
But such criticisms of the specific experiment, although they may be valid, miss the broader point, which brings us to the topic of the burden of proof. It isn’t on me or anyone else to disprove the effect exists — firstly it is on the proponents to provide the proof that it does exist, in the first place. Any physical demonstration of the same principle in a lab-controlled environment. And not only are there no demonstrations, there are actually fraudulent demonstrations. Actual greenhouses don’t work this way though some even today still report that they do, the jar experiments either have doctored results, or even if not, actually demonstrate a different effect, etc… And on top of that, some proponents even say it’s not possible to experimentally prove anyway!
So in a sense my experiment here was all besides the point, but… I just wanted to see for myself, in as best a vacuum as I could easily make, that reflecting a heat source’s own radiation back at, or a heat source heating an object that then radiates its own heat back at it, doesn’t heat up that heat source ![]() .
.
Cheers,
Claudiu
Quantumville doesn’t deal with such dirty realities as air, conduction, convection, breezes, etc… ↩︎ ↩︎
First wire arrangement (horizontal plane) ↩︎
In a vacuum and thus only radiative heat loss we should have:
Surface Area: 193.6 mm^2
Power Input = Power Out: 1.364W
Emissivity: 0.95
Temperature: 328.2°C ↩︎As the foil wasn’t perfectly wrapped the blackbody prediction is anywhere between these two numbers. See next footnote for more detail. ↩︎ ↩︎
With 97% of the energy inside being reflected back in, this back-radiation should heat the heat-source until the amount emitted from the sphere - just 3% of the total power inside - equals the power in.
1.364W / 0.03 = 45.47W , such that 45.47W * 0.03 = 1.364W
Surface Area: 193.6 mm^2
Power Input + Back-Radiation: 45.47W
Emissivity: 0.95
Temperature: 1,178°C ↩︎ ↩︎Second wire arrangement (more looped) ↩︎
By the same logic of the GHE – the wire should initially be emitting 1.364W, which will then heat up the cardboard box. The cardboard box then emits this amount in two directions, 0.682W up and 0.682W down. This 0.682W is added to the 1.364W to get 2.047W, etc., which continues until the equilibrum is reached of the wire emitting 1.364/0.50 = 2.728W
Surface Area: 193.6 mm^2
Power Input + Back-Radiation: 2.728W
Emissivity: 0.95
Temperature: 441°C ↩︎