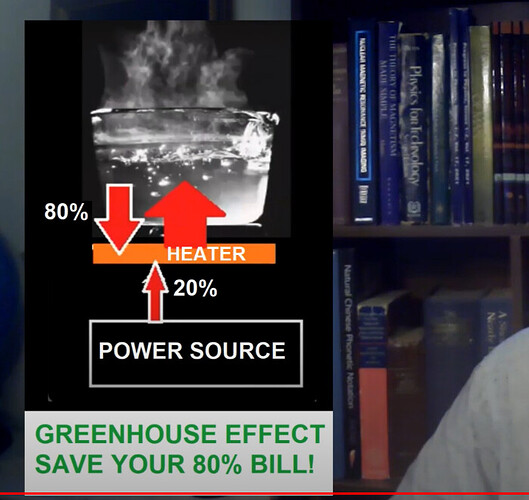
Nice vid and channel – and there at 8:53 comes the Point 11 that I had so much trouble with:
i.e. if downward infrared backradiation did indeed work like it does in the atmospheric greenhouse effect, then this is how stoves would work. There would be a hot surface, and then a small vacuum gap (to prevent conduction & convection), and an IR-transparent pot would sit on top of this. Then the heater surface would be heated to a temperature far less than 100°C. The water would absorb the heat, this would warm the water up, and then radiate infrared both upwards and downwards. The downward infrared radiation would then heat the surface of the heater past what it was (without adding any additional power) until it eventually got to 100°C and boiled the water!
This would be incredibly useful and it surely would have been discovered and invented by now, it would be how stoves work.
Yet in the real world, we have to heat the stove past 100°C in order to boil the water! ![]()
Cheers,
Claudiu